The Royal Ocean Racing Club recommends a minimum of 3l per crew (offshore racing special regulations), per day, but I’ve found that a as a disabled sailor my needs for survival, let alone comfort, are substantially higher. Between the need to stay very much better hydrated than the minimum provides, and the minimum personal cleaning necessary for health, I actually use around 8 litres a day. That forces me to think about on board water supply a lot more than other solo sailors.
Increasingly the standard approach appears to be to use reverse osmosis (a water maker). My purpose is to outline a lower power approach with a filter cascade which because its elements can be separated may be easier to fit, than a typical Reverse Osmosis plant.
I was forced to think about this in more detail by chatting with a sailor who was planning a voyage to some remote areas. This article has grown a lot more than I intended it to – but it now provides a ‘source book’ from which boat owners can pick and choose as much or as little as they need.
Disclaimer
I am an engineer not a public health physician. Please assess the information in this document carefully, and make your own decisions about your needs. If you have doubts, consult qualified professionals. There are no affiliate links in this article and I don’t profit from its distribution.
Why not fit a water maker (reverse osmosis plant)
I know that for many of you the first question would be ‘why don’t you carry a water maker (reverse osmosis) plant’. The unfortunate reality for a lot of very small yachts like Trilleen, is that the power demands are crushing, and finding space for the plant can be a significant struggle.
Designing for clean water success on yachts
In yachts we are in effect, running a micro scale water treatment plant, and given how haphazardly a lot of owners conduct these operations its a bit of a miracle that there aren’t more casualties as a result. This schematic provides one possible arrangement for resolving this problem.
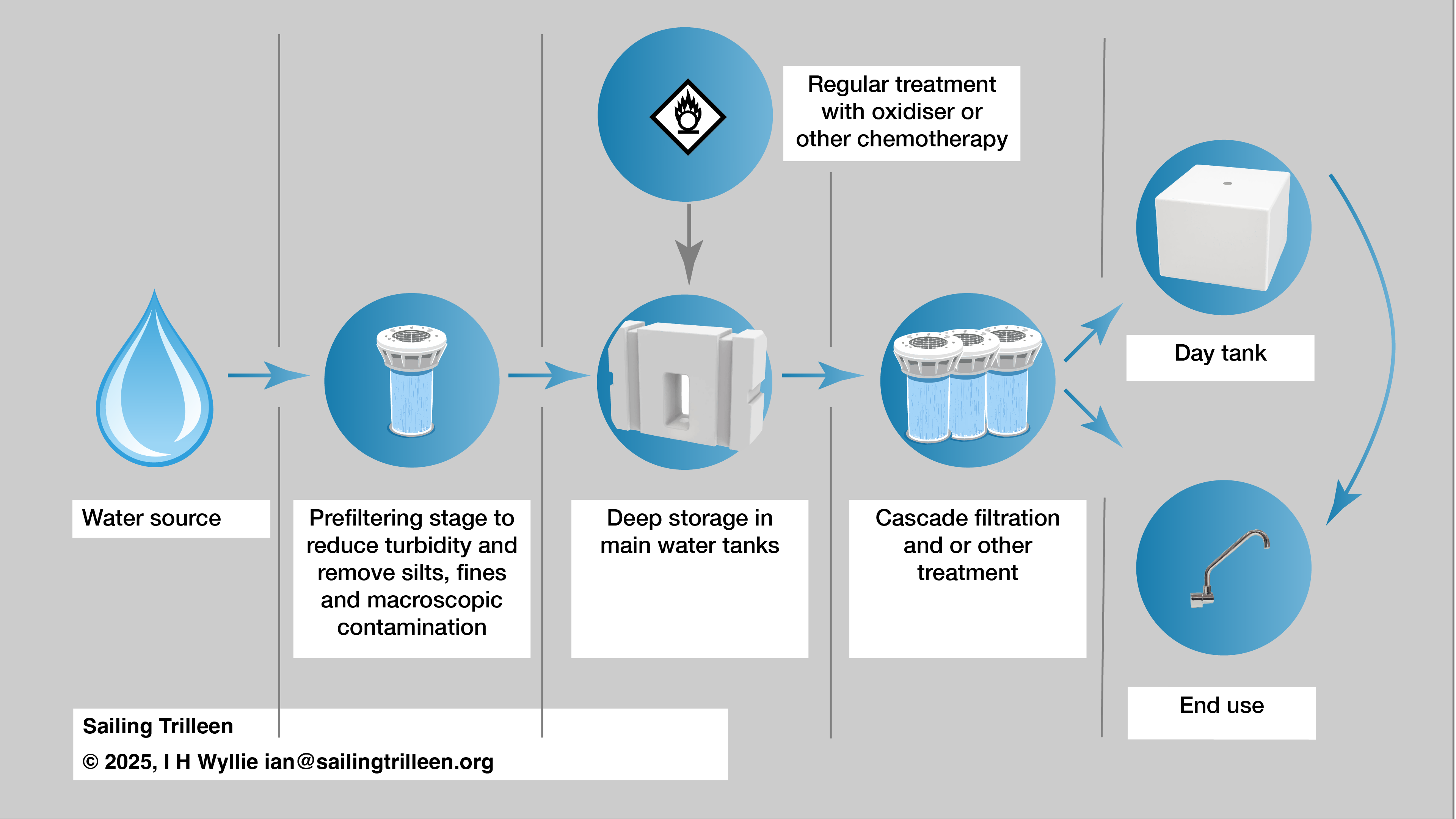
Is the raw water contaminated with metals?
It is essential to restrict collection of water to those supplies which are free from heavy contamination with hazardous metallic elementals (including arsenic), because in small scale water treatment these are impossible to effectively remove. This becomes a particular factor where a yacht is remaining in a single region for longer periods.
Removing bacteria, viri, and protozoa
The next problem is to ensure that bacteria, viri (viruses), protozoa, and dangerous fibres, are either removed by filtration or destroyed by some process.
Removing chemical contaminants
Soluble chemical contamination can be removed – to a degree by filtration with activated carbon.
Safe storage or not?
On yachts we have a further problems in that we typically store water for long periods and use the water at relatively low rates. This is major problem because our tanks are effectively bioreactors in which bacteria can breed. Even municipal supply (tap water) in well developed areas which is likely to contain some bacteria (albeit too few to cause disease) but bacteria breed a lot faster than rabbits. Escherichia coli the well nown faecal bacteria will double around twice at day at 20°C and provided there is sufficient organic material, the number of bacteria will double every twenty minutes at 37°C. This poses particular risks to vessels in tropical and subtropical areas.
What bugs, beasties and nasties are we trying to remove in our water?
We are trying to remove or deal with: coarse contaminants like silt, bacteria, protozoa, viruses, heavy metals, nitrites and nitrites and other chemical contaminants, and finally and hopefully never to be required: radionuclides
What scale are we working at?
The things we are trying to avoid are super small: The useful scale here is millionths of a metre – which engineers usually write as micro metre or micron, and abbreviate as µm. To get a grip on the sizes here, a human hair is usually somewhere roughly in the range 20-150µm, the glass fibres commonly used in E glass GRP are 3-20µm, and many diesel engine filters remove all contaminants down to 10µm.
| Size in µm | Contaminants |
|---|---|
| 10-100 | Suspended fines and silt (very variable depending on substrate), may include insoluble heavy metals. |
| 4-25 | Protozoa Cysts (e.g. those causing destructive eye or CNS disease e.g.(Acanthamoeba, Naegleria , or diarrhea (sometimes long lasting) e.g. Cryptosporidium, or Giardia , ) |
| 1-10 | Bacteria (e.g. those causing: Cholera ( Vibrio cholerae , Typhoid fever (Salmonella typhi) and Bacillary dysentery (varies Shigella, Salmonella, Campylobacter and Escherichia coli) also notable in free water sources: Pseudomonas, Leptospira |
| 0.1-10 | Chrysolite fibres e.g. fumed silica / asbestos, fumed silica (used extensively in boatbuilding) |
| 0.02-0.5 | Viri (Viruses) a selection of those commonly found in ambient water would include rotavirus(es) , possibly Hepatitis E, Adenovirus |
| — | Dissolved heavy metals and elemental materials (e.g Arsenic in parts of the Indian sub-continent, Mekong Delta, parts of California and South America among others). These unfortunately cannot be effectively removed by filtration |
| – | Nitrates, nitrites, other chemicals, radionuclides – cannot be assumed to be effectively removed by filtration, but activated carbon filters are at least partially effective. |
| – | Algae and algal toxins – very variable in size but algae colonies can be effectively filtered. Dissolved toxins may be removed with micro filtration and or activated carbon to some degree. |
How can we remove bugs, beasties and nasties from our water?
Much of the literature from the development community around the protection and treatment of water sources for household and community scale systems is worth reading. These are published by WHO, UNICEF, and national NGO. Search terms include WASH (Water, Sanitation andHygiene)
There are four effective small scale methods which can be combined in complementary ways.
| Method | Bacteria | Protozoa | Viri |
|---|---|---|---|
| Chlorination | ‘+’+’+ | ‘— | ‘+ |
| Filtration <1µm | ‘+’+’+ | ‘+’+’+ | ‘— |
| Filtration < 0.5µm | ‘+’+’+ | ‘+’+’+ | ‘+ |
| UV treatment | ‘+’+’+ | ‘+’+’+ | ‘+’+’+ |
- Chemical treatment: typically with a chlorine based compound. Chlorine, in the right concentration is effective against bacteria, less effective against viri (viruses), and except in very high concentrations is unsafe against protozoa. There is a very good guide to how to chlorinate effectively from the World Health Organisation which is designed to support users in resource challenged situations.
- Filtration to exclusion: typically this requires a cascade of filters which sequentially remove contamination from large to small. Depending on the part of the process, Filters may be, mechanical screens, ceramics, diatomous earths, or carbon based and some of these may be doped with colloidal silver to prevent bacterial regrowth. For a simple guide to primitive filters in the water setting read: Household WaterTreatment Filters. Many filters require back flushing to maintain effectives and it may be necessary to build flushing loops into a yacht system for maximum filter life.
- Boiling: Heating fresh water to a rolling boil water is a very effective way to decontaminate it provided that it is free from fines and turbidity (i.e. has been through a pre-fillter). For some viri and protozoa a more extended boil of up to 10 minutes may be advisable. In practice, except for yachts with very large generation capacity and using induction stoves, this is not likely to be a useful method because of the difficulty in carrying sufficient fuel, and the very significant risks of large quantities of boiling water being regularly in use in a seaway. It may however be of use as an adjunct in the event that other water protection systems fail, that crew become very unwell, survivors are recovered or small children are involved.
- Ultraviolet exposure: Exposure to intense ultraviolet light destroys the DNA of unwanted organisms. Typically this is only effective on water which is substantially already filtered because particulates in the flow can ‘hide’ organisms from the light and permit them to cause infections. Because of this it is usually used as a final stage process. 12v power UV disinfection filters are not common but do exist. Search for Photovoltaic powered UV disinfection, or visit.
- Activated carbon filtration, within the adsorbtion capability of the filter will remove soluble chemical contaminants, which is usually implemented before before the UV disinfection stage.
Can you build a safe boat water system?
Building an effective pre-filter
The purpose here is to ensure that the water in the tank is macroscopically clean – not to ensure that is microbiologically safe, and to prepare the raw water for chemical treatment if you wish to ensure long term safe storage in deep tanks. Ideally this means a turbidity score of around <5NTU (Nephelometric Turbidity Unit (NTU) is a US standard unit) which is visibly very clear water. Turbidity is the ‘haziness’ of the fluid, which appears in fluids much as smoke pollution does in air. A non electronic field measurement technique uses a turbidity tube, which competent boaters could easily construct is described in this paper.
Commercial pre-filters for yachts receiving dockside water
Commercial pre-filters for dock water typically filter at 1 – 3µm (absolute), but do not certify for removal of biologically hazardous elements (e.g. bacteria) despite the filters 1µm claims. These filters appear to be designed to be used with municipal supplies in developed countries, and in such cases are likely to be effective. One major supplier (2025) is General Ecology
Effective pre filters for wild sources
However in the case where cruisers are having to collect water from wells, streams or other sources a 1µm initial filter is likely to be unrealistically restrictive and require continual replacement.

The initial filter will need to be selected against the turbidity of the source. This may mean a progressive series of screens beginning as coarse as 300µm. 300 – 100µm can achieved with reusable stainless mesh screens which remove the need for filter replacement. If you are not regularly collecting wild raw water you may be able to achieve this initial stage with a portable set of screens, rather than having to fit and maintain them permanently.
The ideal target for tank feed water would be to have filtered the raw water using a 1µm much as the commercial dockside filters do. However, depending on the quality of your water source, this may not be achievable in which 3-5µm may be a better target.
How to improve the likely-hood of safe storage in tanks
Chemical treatment of water in tanks is recommended on a regular basis. The cheapest, and most widely available, way of doing this is with Chlorine producing compounds. Chlorination works because Chlorine, an elemental, is an aggressive oxidising agent which destroys critical parts of bacteria – and to a lesser extent viri (viruses). Chlorination is only marginally effective against protozoa.
Chlorine, normally in the form of hypochlorite it is the active agent in bleaches. Typically we measure the concentration of Chlorine in parts per million (ppm), which for all usual doses is equivalent to milligrams per litre (mg/l).
Because Chlorination relies on a series of chemical oxidation reactions to achieve disinfection, effective disinfection depends on Chlorine dose, pH, temperature and time. The UN guide to chlorination provides tools for calculating Ct (Chlorination time), but in general in small sailboats provided that the water has a pH of less than 8, and you have provided sufficient chlorine to meet the demand of the first two oxidation phases (Chlorine demand), see Break Point dosing allowing an hour between dosage and use would be sufficient. Alkaline waters >8pH are undesirable to drink in any case. pH measurement of water is trivial.
There are two methods possible, shock chlorination, and regular, or break point, dosing. For boating purposes Break Point Chlorination would be desirable, with perhaps Shock Chlorination occurring periodically when tanks can be refilled and purged.
Accessing sources of Chlorine
Chlorine is most widely available worldwide in two forms: Sodium Hypochlorite (bleach), and Calcium Hypochlorite (‘powdered Chlorine’) with a typical active chlorine content of 65-75%. In the case of bleach very careful attention should be paid to ensure there are no additives such as fragrances or thickeners. Stabilised Chlorine is sometimes available but must not be used for disinfection of potable water.
Calculating how much chlorine product to add to a tank is not completely straightforward when the raw water quality varies – and in the case of municipal supply account must be taken any pre-existing chlorination.
Break point dosing Chlorination
Regular dosing chlorination is usually called Break Point chlorination in the water industry.
This targets an amount of Free Residual Chlorine in the water of 0.2-0.5 (ppm or mg/l). The only way of achieving this for a sailboat where different qualities of raw water are being added to the tank is to regularly test the product water. Testing is necessary because Free Residual Chlorine only develops after the so called ‘Break Point’ which is preceded by two phases of oxidation. The amount of Chlorine required to reach the break point depends on how much organic matter is in the water (i.e. broadly, how dirty the water is).
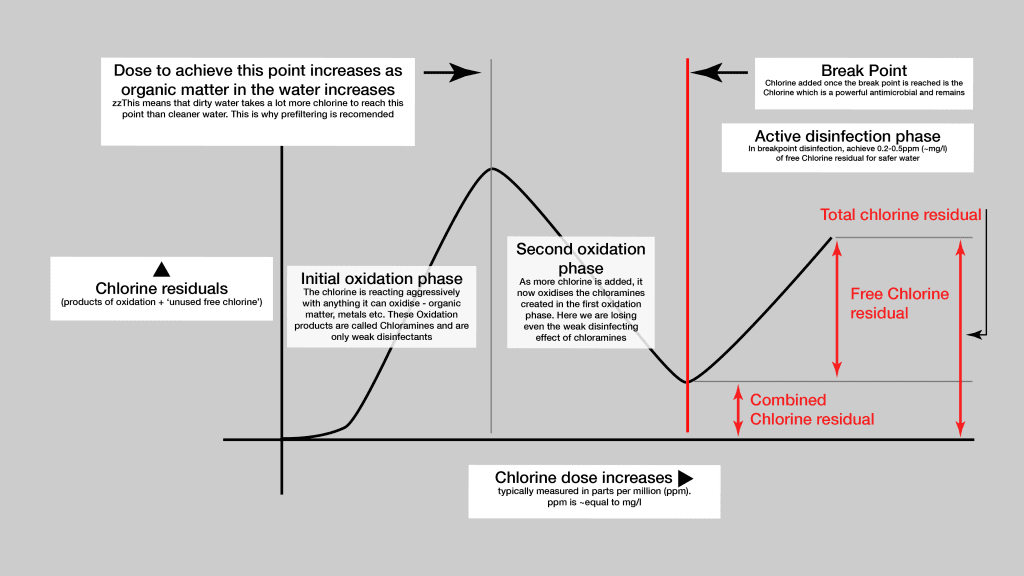
Over time (days) the amount of Free Residual Chlorine will decrease for various reasons. So if a yacht is on a 30 day trans-oceanic passage, it would be advisable to top up the chlorine dose at various points based on measurements.
It should be obvious that in order to achieve effective chlorination, water must pass through to all the delivery points in the boat. This means running faucets for long enough to draw newly chlorinated water through the branch lines, including the necessary volume to refill a calorifier if it is in use. This may be challenging in water poor areas, or if water is being carried by Jerry can. However if the water drawn through the system is collected it can normally be safely fed back into the prefilter stage which may reduce the difficulty.
Shock Chlorination
In the boating context shock chlorination is most useful for periodic thorough disinfection of tanks, and can be effective in countering the build up of biofilms in water systems which can harbour bacteria in the system. The degree to which this is effective depends on how significant the fouling in the tank has become and it is not guaranteed to be effective.
Shock chlorination requires adding a minimum of 200mg/l or ppm to a tank, and then leaving for a period of time. 12 hours would be more than sufficient, then reducing the level of chlorination to safe quantities for consumption. In the boating context this practically means discharging all the water then reloading with fresh water and carrying out the Break Point Chlorination technique.
Protecting the water you drink, cook with and use for teeth washing by building an effective filtration cascade
The most obvious thing to do here is to only protect the water you need to be microbiologically clean for consumption. With a split cascade a 1µm filter can be placed between the tank and all feeds (if this standard has not already been achieved before the tank) This means for example the calorifier or shower, providing water which is fairly safe, but not technically potable, before only further filtering water which is going to be directly drunk or used for cooking.
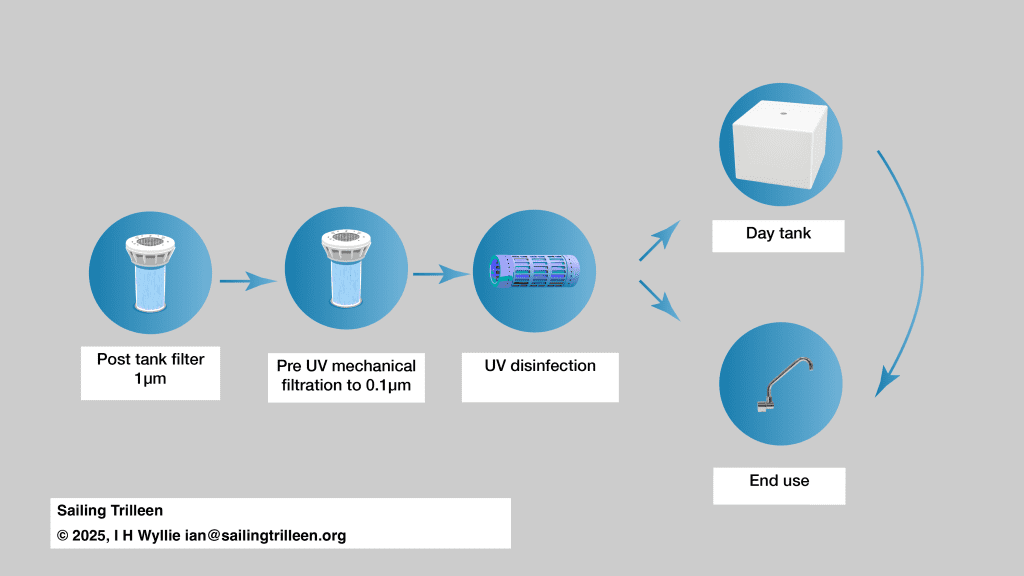
It doesn’t matter how you get there – but the objective is sub micron filtration, ideally 0.1µm but 0.5µm may be the only practical solution for your situation. Given a 1µm filter on the tank exit this should only require one further filter.
Do you require an activated carbon filter stage?
Depending on water sources an activated carbon filter may be advisable. Reputable sourcing of these filtration units is critical if you are relying on them for anything other than taste improvement. They may be particularly valuable if collecting wild run off from agricultural areas or other water sources which could be chemically contaminated. It will also provide cosmetic improvements to water taste.
Implementing a UV light disinfection system
UV light tubes provide disinfection by disrupting the DNA of the organism. They are only certified to provide adequate disinfection for particular flow rates, and for a certain maximum number of hours of operation. They are sensitive and designed for long running operation (ideally always on), which may not be practical on many vessels. Not all UV sources are equally effective, and in the event that the water is not used rapidly some organisms have the capability to ‘self repair’ the damage to their DNA. Read about UV disinfection and reactivation risks
Because of these requirements it may be necessary to batch run the lamp to produce small batches – for example less than one day’s usage, which can be stored in a day tank. Most lamps should not be triggered by a flow switch because they have a warm up cycle. If possible this should be enforced by inserting a cycle timer to prevent a pump moving water to the day tank before the lamp is fully active.
If possible ensure that the system you purchase is specifically designed to work at the voltage your boat runs on. It is highly advisable to supply this critical equipment via a DC-DC converter to ensure stable voltage.
Don’t protect more than you need
While you could protect the entire boat, there may be problems with ensuring that water left in lines remains clean. Suppling filtered water to calorifiers and expecting it to stay ‘microbiologically clean’ is not likely to be successful.
Warning: Filtration below 5µm removes free residual chlorine and most chlorine oxidation products.
Considerations for choosing a water source
In choosing a safe source you may find it useful to read “Water Safety Planning for Small Community Water Supplies” from the United Nations. This will allow you to assess how safe an individual source is by looking at how well local people manage their infrastructure.
Choosing a water source requires understanding of where the water is coming from, and it is the subject of large publications.
In decreasing order of reliability…
- Treated, well managed municipal supply
- Rain catch
- Deep borehole groundwater
- Direct springs in rock formations
- Unmanaged or poorly managed municipal supply
Sources which should be assumed to be contaminated but may be usable
- Deep village wells Well maintained deep village wells are generally good water sources but should be assumed to be contaminated
- Rainwater catch tanks and cisterns in villages
- Springs where the level of the spring varies directly with precipitation
Avoid the following sources if possible
- Surface water
- Impounded water
Rain catch
Rain and clean snow melt is effectively distilled water. However it is not risk free because contamination occurs from collection surfaces. Pipes and rain catch surfaces on boats are very likely to be colonised by bacteria, however carefully they are cleaned, and it is possible that viri (viruses) will also sneak in from avian faecal contamination, or through poor hand hygiene by handing humans. Contamination is however possible in areas of high airborne pollution (e.g. near wildfires, around coastal industrial plants, ship breaking areas, or downwind of major smog events. This may include heavy metals, and carcinogens.
Boreholes
Direct feeds from boreholes into ground water are possibly safe providing the pipework has been properly maintained. Unless you maintain a trusted chain of custody for borehole water, ideally in your own tanks, assume that water has been transferred via contaminated fixed or mobile tanks and that contamination has occurred – especially if water is distributed in animal (e.g. donkey / goat etc.) pulled carts.
Natural, rain invariant, springs
A degree of geological knowledge is necessary to assure yourself that this is actually deep groundwater coming to the surface. However if you can secure the water into a clean catch basin at the point the spring exits these are can be very good sources. The same assurance cannot be granted about rain fed springs.
How could I test my water?
Most systematic water testing is beyond the capacity of a small yacht. Regular careful maintenance is likely to be the best protection. The exception will be where the main storage tank is being maintained with Chlorine.
Testing for PH
pH testing is trivial either with the use of colometric paper systems or electronic testers. In the case of measuring pH for water testing it is likely you are only interested in whether the pH is acceptably neutral and so lab grade equipment is not required.
Testing for Chlorine concentrations
The use of a DPD test (N,N-diethyl-p-phenylenediamine) colorimetric test. DPD tests are sometimes called Palintests, after the chemist who discovered the process. These can be carried out with ease and is available worldwide. Be careful to select the right sort of test, or at least to understand what you are testing. There are variants which test specifically for Free Residual Chlorine and those which test for other chlorination parameters. Reagents for this testing are often available in tablet form, and these are heat stable.
Measuring turbidity
While electronic measurements of turbidity are widely available their expense may not be justified. A turbidity tube is a water column with a Secchi disk. Measuring the height of water column needed for the Secchi disk to disappear is a crude method of assessing turbidity. For this purpose the a water column of 90cm is required. Tables of correlation between distance and turbidity in NTU or other units are available.
Testing for Heavy metals
Screening testing strips for common heavy metals are affordable and can be carried if wild water sources are likely to be necessary. Arsenic compound testing strips are also available if you are venturing to areas where this is a likely risk. These areas include but are not limited to parts of Argentina, Bangladesh / Ganges delta, California (USA), Chile, Mexico, Vietnam (Mekong Delta).
Testing for the presence Arsenic
Screening testing strips for Arsenic contamination are also availble
What if anything can Total Dissolved Solids show?
TDS meters have become common equipment in the yachting community, Except in the case of reverse osmosis they are such a general measure that they may not be meaningful. They do not tell you what the dissolved solids are, and are certainly no protection against bacterial contamination.
In the case of reverse osmosis they make sense because of the assumption that the input is clean seawater – and therefore any dissolved solids in the product water is residual NaCl and a measure of membrane breakdown.
Very unusual contamination problems
Radiological contamination following nuclear accidents or explosions is a potentially significant risk. Most recently this occurred after Fukishima, and previously Chernobyl, Windscale, and Three mile island. The behaviour of radionuclides during water treatment has been studied.… Some, but not all radionuclides can be attenuated by the use of activated carbon filters.